Ever notice how drugs are so strictly regulated but the surgical innovation field is a bit of a free-for-all?
When Dr. Hani Marcus was training as a neurosurgeon-scientist, his PhD explored how robotics could be applied to minimally invasive surgery in the brain. While developing new technology, he quickly ran into a problem: there was no established way to evaluate whether these innovations were safe and effective for patients.
Drug development, on the other hand, has long benefited from a well-defined sequence of phase I to IV clinical trials that guides new pharmaceuticals from their first use in humans through to long-term monitoring. Yet when it comes to surgical innovation: why have we never seen an equivalent?

Historically, new surgical techniques and devices have advanced through trial, error, and iteration. With surgery being a complex intervention, new innovation can be dependent on the individual surgeon. They may refine a new technique, publish their experience, and over time, others adopt it organically. This lack of structure prompted the creation of IDEAL: a framework providing a consistent, ethical, and evidence-based approach to evaluating new surgical procedures and technologies.
Why IDEAL Exists
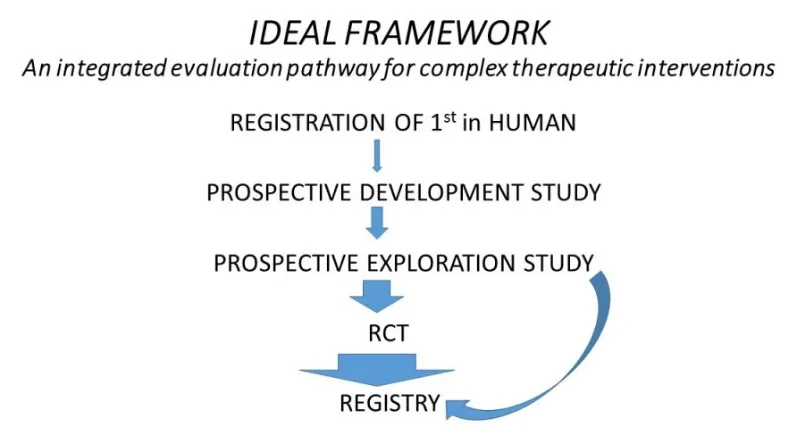
IDEAL stands for Idea, Development, Exploration, Assessment, and Long-term study. It was developed at the University of Oxford almost 20 years ago, bringing together ethicists, clinicians, and researchers.
As the recently appointed Chair of the IDEAL Collaboration, Dr. Hani Marcus has played a pivotal role in shaping how IDEAL evolves to keep pace with emerging technologies like artificial intelligence (AI) and robotics. His work spans both clinical practice — where his team was the first in the world to introduce AI-assisted coaching in pituitary surgery — and academic research, where he focuses on two questions: how can AI help surgeons make decisions, and how can robotics improve surgical execution?
“Technology development and technology evaluation are equally important. It is easy to build something and assume it will be transformative. Proper evidence is the only way to remain honest about whether it truly works.” – Dr. Hani Marcus.
The IDEAL framework helps innovators do just that. Its central insight is simple but powerful: what you need to measure about a new technology will change over time.
What the IDEAL Stages Mean
- Idea: The initial concept of a new surgical technique, device, or AI.
- Development: Refinement of the technique or device through iterative modifications and small case series.
- Exploration: Broader use by other surgeons or centers, often in prospective cohort studies or registries. This stage aims to identify key variables, standardize procedures, and prepare for formal evaluation.
- Assessment: Formal comparative evaluation using randomized controlled trials or other studies to determine safety, efficacy, and effectiveness relative to standard care.
- Long-term study: Ongoing monitoring of outcomes, complications, and evolving impact after the innovation is integrated into standard practice.
Bringing IDEAL Into AI and Robotics
Dr. Marcus has played a leading role in extending IDEAL to new frontiers. He authored IDEAL Robotics guidelines, published in Nature Medicine, and contributed significantly to Decide AI, a set of recommendations for evaluating AI in healthcare. These frameworks are already informing how regulators think about surgical innovation, even if adoption remains early.
“These frameworks are used far beyond pituitary or neurosurgery,” Dr. Marcus says. “They apply to any AI or robotics technology in any part of medicine. That’s what makes them so impactful.”
The IDEAL collaboration now works with regulatory bodies such as the Food and Drug Administration (FDA) in the United States, the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK, and the European Medicines Agency (EMA). While IDEAL isn’t legally mandated, adherence to its principles is a sign of best practice.
Advice for Innovators
For surgeons and engineers developing new devices, Dr. Marcus offers simple guidance: engage early.
“The first thing I tell people is to get in touch with us. IDEAL has an advisory service that helps teams align their projects to the right stage,” he explains. “It’s about asking the right questions at the right time — what to test, when to test it, and how to test it safely.”
IDEAL is not static. It continues to evolve as technology changes. As AI and robotics become deeply embedded in medicine, the framework adapts to ensure innovation remains safe, evidence-based, and centered on patients.
Coming next: In Part 2, we will be exploring how Dr. Marcus and his team are bringing these principles to life in their practice — from AI-assisted coaching in pituitary surgery to overcoming the barriers that still limit the adoption of surgical data science worldwide.



.png)
.png)